EyeOP1®, ETC’s product, was approved by CFDA in October 2017, and the company will focus on clinical and academic promotion in 2018. ETC actively promotes UCP technology and obtains support from authoritative professors at home and abroad. Famous academic leaders, experts and professors at home and abroad gave keynote speeches at the ophthalmologists’ conference of Zhejiang medical association, the annual ophthalmological conference of France, the national academy of ophthalmology and other authoritative industry conferences, and popularized UCP technology to attendees, which was widely recognized by the industry. By the end of the second quarter of 2019, EyeOP1® products and technologies were successfully used by more than 1100 patients from more than 120 general hospitals or eye hospitals in China, with remarkable results and good postoperative follow-up. Several top three grade A class hospitals in China gradually began the equipment bidding process and then they will purchase the equipment and instill them in the hospital.

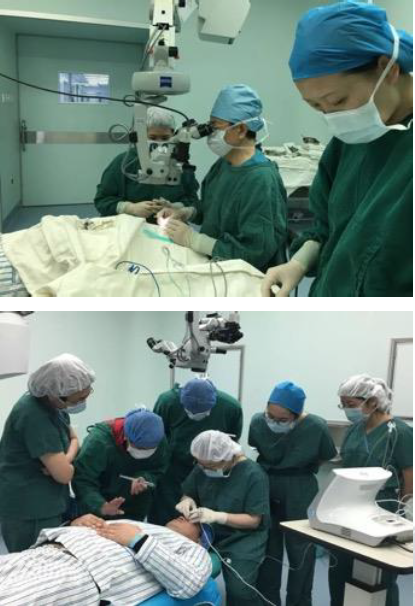
Application of UCP technology in Qingdao Eye Hospital
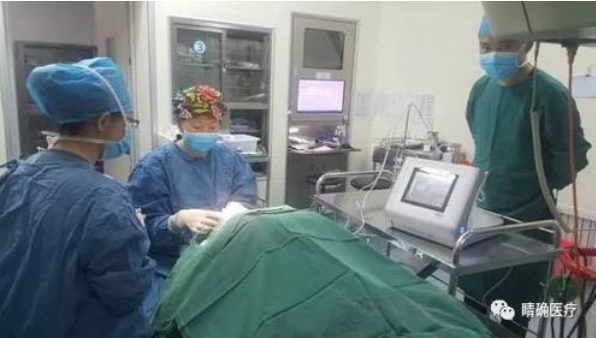
Application of UCP technology in First Affiliated Hospital of Xi 'an Jiaotong University
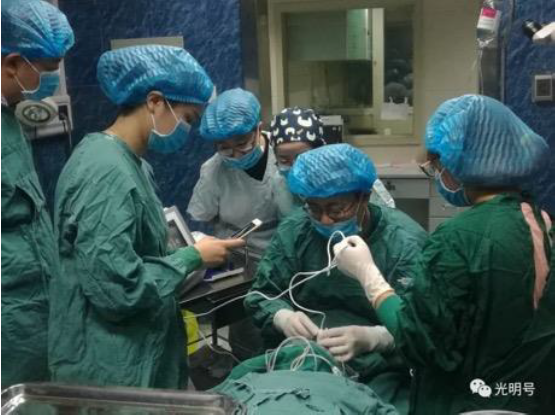
Application of UCP technology in First Affiliated Hospital of Kunming Medical University
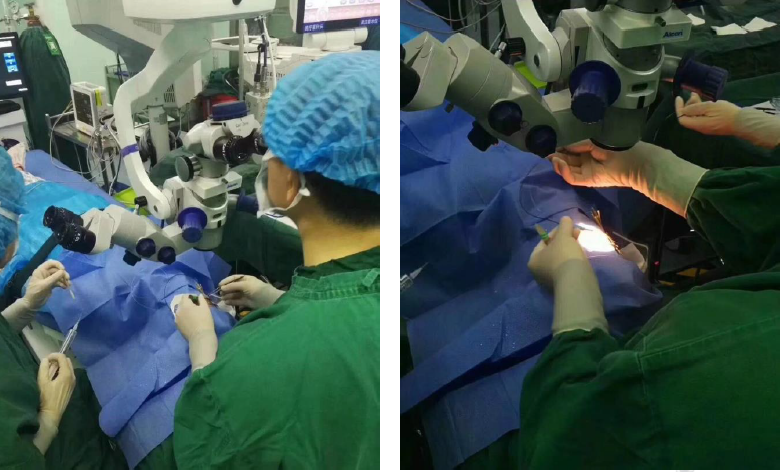
Application of UCP technology in Shenyang Aier Ophthalmology
Application of UCP technology in Changsha Honglang Eye Hospital

Together with Kuntin Group, Everpine Capital established an industrial park fund to seek investment opportunities in the public Pre-REITs market
Updated By 2021-10-09
Recorded in The Glaucoma Surgery Book! High-Intensity Focused Ultrasound Ciliary Body Plasty(UCP) Has Been Recorded in The Glaucoma Surgery Book “ Illustrated Surgical Techniques and Pearls of Glaucoma”
Updated By 2019-08-19
The 2019 Summer Camp held by MacDuffie Shanghai and MIT Opens
Updated By 2019-08-08
More Than 90% Graduates accepted by The World's Top 100 Universities, The MacDuffie School Reached a Record High in 2019
Updated By 2019-05-31