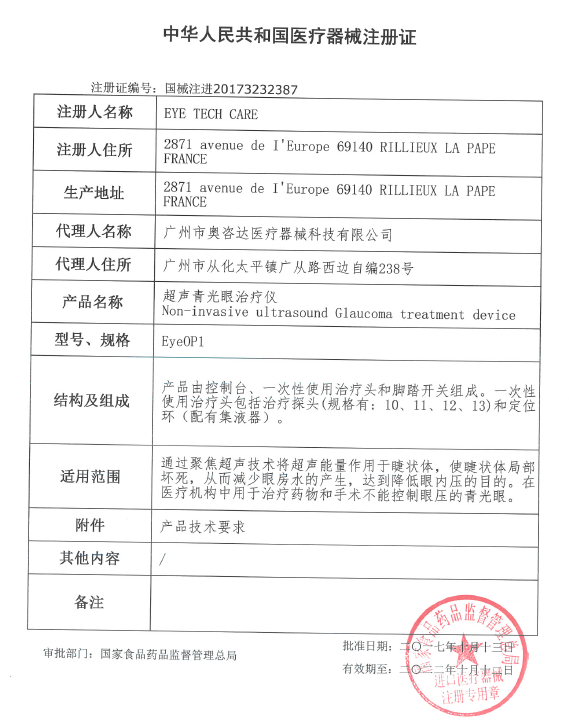
Award-winning medical technology company EYE TECH CARE received approval from China's food and drug administration (CFDA) on October 13 to begin selling its EyeOP1 (R) glaucoma device in China.
As the first non-invasive medical device approved for the treatment of glaucoma in China, EyeOP1® innovatively uses high-intensity focused ultrasound technology to treat glaucoma. To demonstrate the benefits of the technology, EYE TECH CARE has conducted a number of clinical studies, including in China, treating 5,000 patients.
Dr Dietrich Wolf, chief executive of EYE TECH CARE, said: "we are delighted to have received approval from China food and drug administration, which is an important milestone. We are confident that our technology will bring high value to the Chinese glaucoma market and provide new treatment options for patients. With the support of our Chinese and European shareholders, we believe this is a good example of cross-border cooperation between China and France and the beginning of a bright journey for our company. We are now working hard to prepare for the launch of Chinese products."

Together with Kuntin Group, Everpine Capital established an industrial park fund to seek investment opportunities in the public Pre-REITs market
Updated By 2021-10-09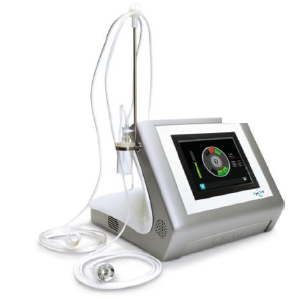
UCP Glaucoma Surgery Technology Has Been Widely Recognized in The Ophthalmology Industry, Covering More Than 120 General Hospitals or Specialist Eye Care Hospitals in China
Updated By 2019-08-19
Recorded in The Glaucoma Surgery Book! High-Intensity Focused Ultrasound Ciliary Body Plasty(UCP) Has Been Recorded in The Glaucoma Surgery Book “ Illustrated Surgical Techniques and Pearls of Glaucoma”
Updated By 2019-08-19
The 2019 Summer Camp held by MacDuffie Shanghai and MIT Opens
Updated By 2019-08-08
More Than 90% Graduates accepted by The World's Top 100 Universities, The MacDuffie School Reached a Record High in 2019
Updated By 2019-05-31